Molecular Orbital Diagram For S2
Molecular orbital theory Orbital atomic nodes 2s 3s 1s atom nodal probability classnotes Need a molecular orbital diagram for s2, s2+ and s2- not the
Molecular Orbital Theory | Grandinetti Group
Shapes of atomic orbital Molecular orbital electrons 3s 3p partially filled solved Molecular orbital diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level cl2 libretexts electron delocalized second homonuclear row
Orbital molecular mo o2 diagram theory orbitals oxygen bond order configuration paramagnetic electrons energy diagrams unpaired two draw chemistry which
Orbital orbitals orbitali shown molecolari determine 2s corresponding ibridiChapter 6.5 delocalized bonding and molecular orbitals Molecular orbitals bonding diagram mo energy complete ao bond order li2 electrons chemistry ion delocalized has unpaired geometry chemwiki exercisesWhat is the difference between bonding and antibonding molecular.
Molecular orbitals in carbon monoxide coMolecular orbital diagram carbon monoxide orbitals configuration energy level electronic chemistry mo theory bonding explanation bond fluorine nitrogen questions shapes Orbital 2s 3s differ does socratic orbitalsOrbital molecular diagram cl2 s2 molecule mot unpaired orbitals electron bond bonding draw c2 molecules mo energy theory valence electrons.

Molecular orbital diagrams simplified
Chem orbitals shapes quantum chemistry model atoms using theory orbital diagram electrons spherical sublevels figure wave mechanics shapedMolecular orbital theory Orbital nodes orbitals 1s atomic chemistry shape shapes 2s node atom electron structure table radial 3s periodic vs representation chem8.4: molecular orbital theory.
Atomic theoryInorganic chemistry Molecular orbital diagram orbitals cl2 atomic bonding energies bond delocalized theory energy according np ordering atoms determine chemistry molecules chemwikiHow does the 3s orbital differ from the 2s orbital?.

Orbitals electron atomic atom structure orbital does shapes energy model chemistry atoms modern there elements electrons levels many level configurations
Orbital molecular diagram b2 theory libretexts chemistry energy chem bond electrons order bonding deki api revision unpaired8.3 development of quantum theory – chem 1114 – introduction to chemistry Orbital calculationOrbital molecular simplified.
Question #84799 + exampleBonding molecular between antibonding orbital orbitals mo bonds theory difference covalent pi diagram electron energy ethylene chemistry polyatomic anti multiple Shapes of atomic orbitalSolved consider the partially filled-in molecular orbital.

Chapter 6.5 delocalized bonding and molecular orbitals
.
.


Shapes of atomic orbital | Chemistry, Class 11, Structure Of Atom

Molecular Orbital Theory | Grandinetti Group
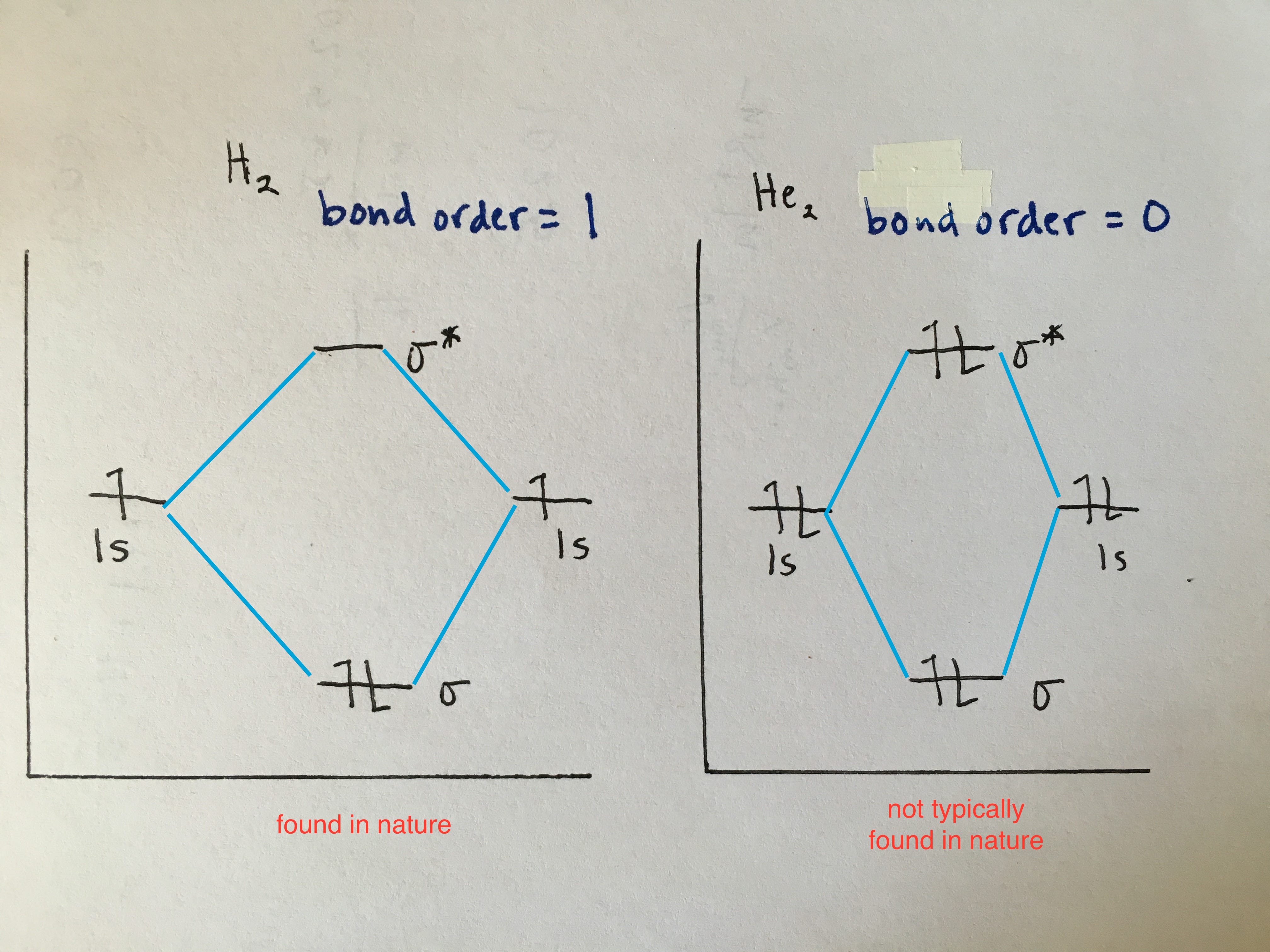
Molecular Orbital Diagrams simplified | by Megan Lim | Medium

Shapes of Atomic Orbital - Chemistry, Class 11, Structure of Atom

Solved Consider the partially filled-in molecular orbital | Chegg.com

What is the difference between bonding and antibonding molecular

How does the 3s orbital differ from the 2s orbital? | Socratic

Molecular orbitals in Carbon Monoxide CO